| Effective communication channels like toll-free numbers and websites have been put in place for various stakeholders to report adverse drug reactions (ADRs). Our medical representatives also act as an interface with our stakeholder spectrum and are trained to capture adverse events. In certain geographies, drug safety information is also received directly from Regulatory Health Authorities. |
|
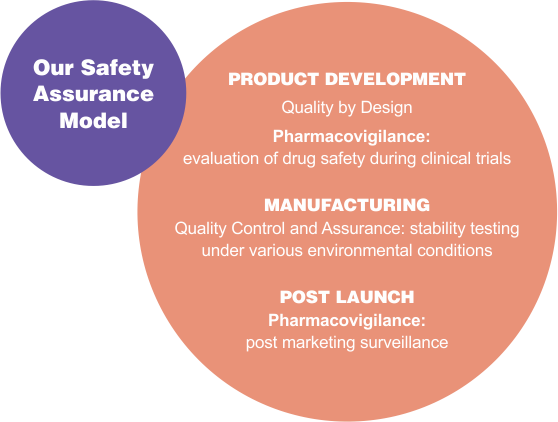
All reported adverse events are continually and systematically aggregated and reviewed by pharmacovigilance drug safety physicians for untoward drug safety signals. Such an approach enhances customer satisfaction, ensures patient well-being and addresses regulatory notifications. In the reporting year, we established an electronic gateway
with the US FDA for submission of adverse event reports. |
|
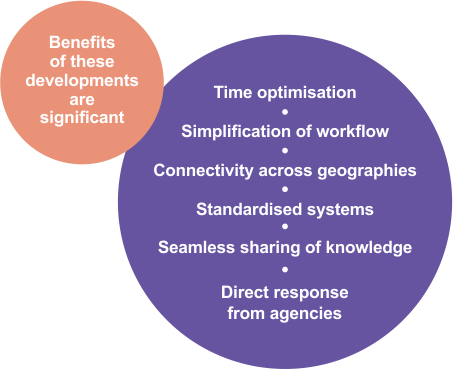
The gateway allowed pharmacovigilance to change from a paper-based submission process to an electronic one. We also migrated our safety database to a higher version thus ensuring compliance with the changing global drug safety reporting regulations and creating a common platform for sharing safety information for the PV staff stationed across geographies. |