|
|
| |
| |
| ECONOMIC PERFORMANCE |
| AFFORDABLE & INNOVATIVE MEDICINE |
|
|
| Proprietary Products |
| The third pillar along with affordability and accessibility that fulfils our motto of 'Good Health Can't Wait', is availability. In spite of leaps that we have taken in the world of medicine, there are still many peaks to be conquered – many cures to be discovered for untreated diseases and many strides to be taken to reduce the cost of medicines. And this involves making available new molecules, advanced technology and novel formulations. |
| Thus, our Proprietary Products segment is geared towards discovery and development of new chemical entities and differentiated formulations for subsequent commercialisation. This segment also includes our dermatology focussed specialty business operated through Promius™ Pharma. |
|
We continue to leverage our semi-virtual research and development model to expand our portfolio of drug discovery, and differentiated and specialty formulations programmes. We achieve this by efficiently collaborating with different biotechnology companies and service providers, and tapping their expertise in the drug development process. We continued to progress towards building a sustainable mix of proprietary, branded research and development portfolio with significantly reduced fixed costs. |
|
| |
| Proprietary Product Folio |
New Chemical Entities
(NCEs) |
| The discovery and development of new small molecule agents in different areas, especially therapeutic ones such as bacterial infections, metabolic disorders and pain & inflammation. |
|
|
| Specialty Business |
| A portfolio of products that includes in-licensed patented dermatology products and off-patent cardiovascular products. We also have an internal pipeline of dermatology products that are in different stages of development. |
|
|
| Differentiated Formulations |
| Our Differentiated Formulations division consists of new, synergistic combinations as well as technologies that improve safety and/or efficacy by modifying pharmacokinetics of existing medicines, focussed on significant clinically unmet needs. We are also investigating new indications for existing medicines. |
|
|
| |
| Innovation Model |
| Our research and development efforts have a unique 'medicines-to-molecules' approach to product development. In this approach, we leverage in an integrated manner, the disciplines of biology, chemistry, drug delivery, clinical development, regulatory and commercial positioning to construct novel differentiated formulations and NCEs. |
|
| We follow a hybrid research and development model, both in-house and virtual (where operations are outsourced, subject to our retention of strategic and project management functions), with the following core principles: |
 |
Develop creative research and development investment models and partnerships to tap external innovation focussed on leveraging, rather than replicating, unique core competencies. |
|
 |
Select assets based on potential for early risk mitigation, both with respect to product development and commercialisation. |
|
 |
Leverage knowledge and presence in emerging markets (especially India) to maximise cost advantage. |
|
| |
| Our principal research laboratory is based in Hyderabad, India. As of March 31, 2015, we employed a total of 152 scientists pursuing an integrated research strategy through a mix of translational, formulation and analytical research at our laboratories. Our research strategy focusses on the discovery of new molecular targets, designing of screening assays to screen promising molecules and developing novel formulations of currently marketed drugs, or combinations thereof, to address unmet medical needs. |
| While we continue to seek licensing and development opportunities with third parties to further develop our product pipeline, we also conduct clinical development of some candidate drugs ourselves, which will enable us to derive higher value for our products. Our goal is to balance internal development of our own product candidates with in-licensing of promising compounds that complement our strengths. We also pursue licensing and joint development of some of our lead compounds with companies looking to implement their own product portfolio. |
|
|
| |
| Pipeline Status |
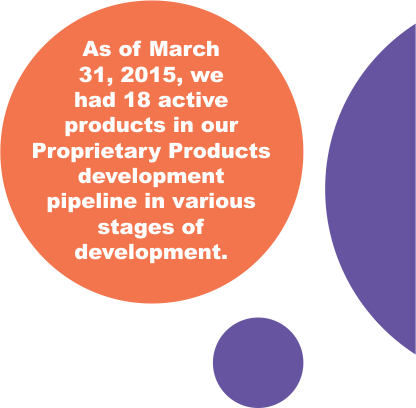
| As of March 31, 2015, we had 18 active products in our Proprietary Products development pipeline in various stages of development. We have filed 3 NDAs for products from this pipeline, and two in March 2015. |
| The details of our active products which are in Phase III or for which an NDA has been filed from our Proprietary Products segments as of March 31, 2015 are as follows: |
| Compound |
|
Therapeutic
Area |
|
Status |
|
Remarks |
| DFN-02 |
|
Migraine |
|
Phase III / 2018 |
|
Targeting migraine with or
without aura |
| DFD-01 |
|
Psoriasis |
|
U.S. NDA submitted |
|
Targeting plaque psoriasis |
| DFD-06 |
|
Psoriasis |
|
Phase III / 2016 |
|
Targeting moderate psoriasis |
| DFN-11 |
|
Migraine |
|
U.S. NDA submitted |
|
Targeting acute migraine |
| DFD-09 |
|
Dermatology |
|
U.S. NDA submitted |
|
Targeting inflammatory lesions |
|
|
| |
| Patents Dashboard |
Our Proprietary Products segment had the following patents filed and issued as of
March 31, 2015: |
|
|
| Category |
|
USPTO(1)
#Filed |
|
USPTO(1)
#Granted |
|
PCT(2)
#Filed |
|
India
#Filed |
|
India
#Granted |
| Anti-diabetic |
|
85 |
|
17 |
|
62 |
|
117 |
|
45 |
| Anti-cancer |
|
18 |
|
11 |
|
14 |
|
45 |
|
15 |
| Anti-bacterial |
|
8 |
|
7 |
|
10 |
|
22 |
|
4 |
| Anti-inflammation |
|
43 |
|
23 |
|
35 |
|
24 |
|
3 |
| Anti-ulcerant |
|
1 |
|
1 |
|
- |
|
1 |
|
- |
| Miscellaneous |
|
11 |
|
3 |
|
3 |
|
26 |
|
8 |
Differentiated
formulations |
|
16 |
|
1 |
|
13 |
|
12 |
|
- |
| TOTAL |
|
182 |
|
63 |
|
137 |
|
247 |
|
75 |
|
|
| Note: |
| (1) USPTO means the United States Patent and Trademark Office. |
| (2) PCT means the Patent Cooperation Treaty, an international treaty that facilitates foreign patent filings for residents of member countries when obtaining patents in other member countries. |
|
|
| |
| Promius Pharma |
| Promius Pharma is our subsidiary in Princeton, New Jersey in the United States focussing on our U.S. Specialty Business, which is engaged in the development and sales of branded specialty products in the therapeutic areas of dermatology and neurology. It has a portfolio of in-licensed patented dermatology products. |
|
|
| It also has an internal pipeline of dermatology products that are in different stages of development. Promius Pharma's current portfolio contains innovative products for the treatment of seborrheic dermatitis, acne and steroid responsive dermatoses. It has commercialised five products: |
|
|
 |
EpiCeram®, skin barrier emulsion for the treatment of atopic dermatitis |
|
 |
Scytera™, foam for the treatment of psoriasis |
|
 |
Promiseb™, cream for the treatment for seborrheic dermatitis |
|
 |
Cloderm® (Clocortolone Pivalate 0.1%), cream used for treating dermatological inflammation |
|
|
 |
Trianex®, cream for the treatment of the inflammatory and pruritic manifestations of corticosteroid responsive dermatoses |
|
|
|
Promius Pharma leverages our research, development and manufacturing facilities in Hyderabad, India. Promius Pharma also works with various third party research organisations in conducting product development, pre-clinical and clinical studies. The manufacturing of Promius Pharma's products has been outsourced to third party manufacturers based in the United States and Europe. |
|
| |
|
|